
Surmodics Provides Regulatory Update Related to its FDA Premarket Approval Application for the SurVeil™ Drug-Coated Balloon | Business Wire

FDA Approves Bard's Lutonix 035 DCB For Dysfunctional AV Fistulae Treatment | Medical Product Outsourcing
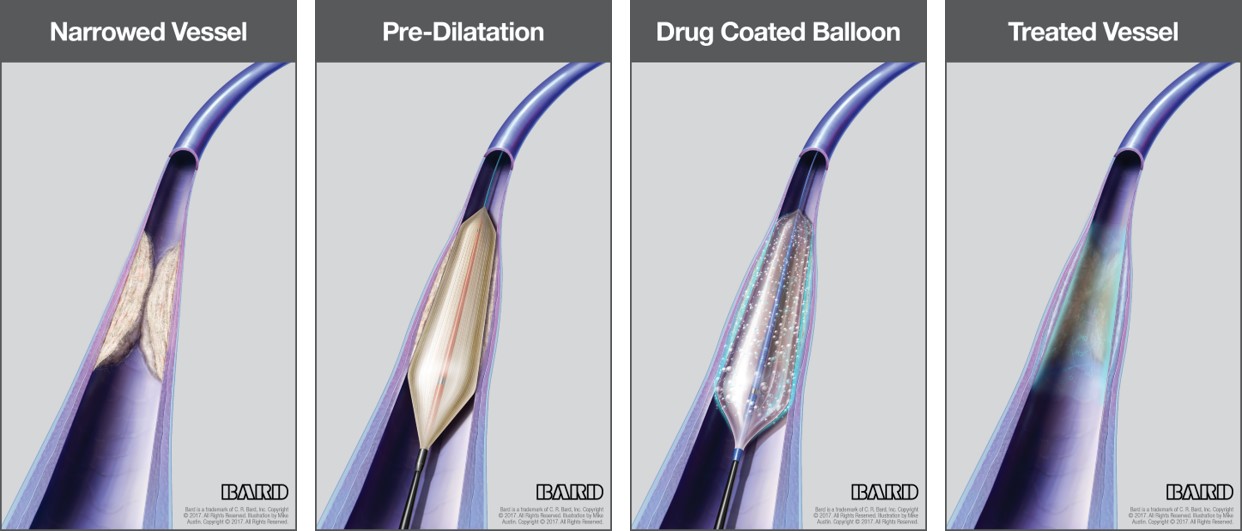
C. R. Bard Receives FDA Premarket Approval For The LUTONIX 035 Drug Coated Balloon - Medical Design and Outsourcing

FDA Approves Medtronic's 200 & 250mm IN.PACT Admiral Drug Coated Balloons | Medical Product Outsourcing

Advanced NanoTherapies' SirPlux Duo Drug-Coated Balloon Receives FDA Breakthrough Designation for Small Vessel Coronary Artery Disease

Chocolate Touch drug-coated angioplasty balloon for treatment of PAD receives FDA approval - Vascular News
MedAlliance's SELUTION SLR drug-eluting balloon (DEB) receives FDA investigational device exemption (IDE) approval, making it the first limus DEB to be available to US patients
Concept Medical received IDE approval to investigate safety and efficacy of its MagicTouch Sirolimus Coated Balloon Catheter for the treatment of small coronary artery disease | tctmd.com



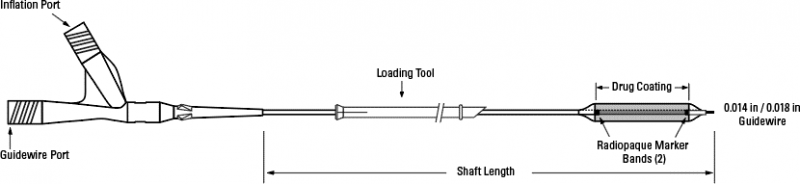


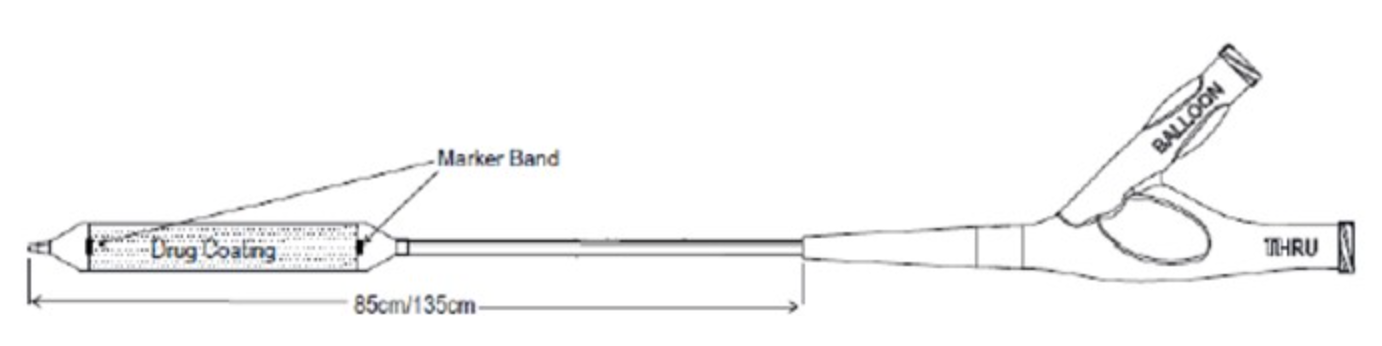









_1653668541.jpg)

